“We finally have a vaccine against malaria and that in itself shows this can be done and that funding and research must continue because it’s possible”— Mary Hamel, WHO lead for the Malaria Vaccination Implementation Programme
Reporting for Devex from Ivory Coast is Christin Roby, its West Africa Correspondent covering global development trends, health, technology and policy-related topics for it. In this report originally titled “Behind the scenes on new malaria vaccine pilot roll-out” Roby and Devex post a narrative that suggests an impending crushing of malaria at last. Roby, a product of a Masters in Journalism, with reference to Videography and Global Affairs Reporting from the Medill School of Journalism at Northwestern University is tweetable @robyreports
By Christin Roby
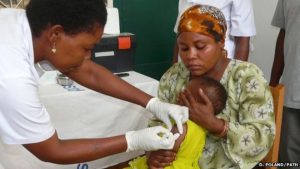
The United Nations Children’s Fund will be the leading partner in the pilot implementation of the first-ever malaria vaccine in Ghana, Kenya, and Malawi, announced by the World Health Organization on Monday, (24/04/2017). The observational program and study is meant to provide evidence about the vaccine’s efficacy in real-life settings. The information in the pilot will be key in deciding whether to deploy the vaccine, known as RTS, S, on a wider scale.
One powerful shot somewhere in Tanzania
One of the key questions is surrounding the feasibility of RTS,S in countries with weak health systems. The vaccine is given in four doses, spread over a period of time. Researchers will be watching whether it is possible to effectively track patients and ensure they return for repeated doses. “Vaccines that require a multiple dosing schedule require considerable additional logistics, community sensitization and communication to explain why to return for the additional shots,” UNICEF’s Global Malaria Advisor Valentina Buj told Devex.
Targeting more than 750,000 young children, the WHO will also examine the effectiveness of the RTS, S malaria injection to assist immune systems in fighting off the malaria parasite most commonly found in sub-Saharan Africa, Plasmodium falciparum. The implementation period will assess the impact on overall childhood survival, and monitor closely adverse effects from the medicine. Advocates hope the vaccine will provide a major boost for efforts to end malaria, which in 2015 infected 212 million new patients and caused some 429,000 deaths, mostly young children in Africa, according to the WHO. “We definitely need more tools beyond the bednets and good case management and the control methods used now,” David Kaslow, vice president of product development at PATH told Devex. “It’s clear we still need more tools particularly in areas where there is high malaria burden.”
Roll-out
 The ministries of health in the three pilot countries will lead the trial’s roll-out, including deciding which districts and regions to include, with priority given to high malaria burden areas. UNICEF will work alongside authorities to develop a plan, for example by sharing lessons learned from previous vaccination roll-outs, according to Buj. In Kenya, UNICEF will be responsible for monitoring the supply chain that delivers the vaccine to children and for documenting the introduction process, UNICEF health specialist Peter Okoth told Devex. The agency’s Kenya country office will also help develop a communications strategy to reach out to child caregivers. Targeted areas will likely include those also supported by maternal and child health government services in the past.
The ministries of health in the three pilot countries will lead the trial’s roll-out, including deciding which districts and regions to include, with priority given to high malaria burden areas. UNICEF will work alongside authorities to develop a plan, for example by sharing lessons learned from previous vaccination roll-outs, according to Buj. In Kenya, UNICEF will be responsible for monitoring the supply chain that delivers the vaccine to children and for documenting the introduction process, UNICEF health specialist Peter Okoth told Devex. The agency’s Kenya country office will also help develop a communications strategy to reach out to child caregivers. Targeted areas will likely include those also supported by maternal and child health government services in the past.
The WHO lead for the Malaria Vaccination Implementation Programme, Mary Hamel, told Devex that the major challenge will be understanding the most effective dosing schedule. “I am confident that we will find out how best to deliver the doses including a fourth dose, and whatever we find that works will be sustainable,” she said.
If successful, the vaccine could be a powerful tool in the WHO’s global technical strategy for malaria which aims to reduce malaria case incidence and death rates by at least 90 percent, eliminate the disease in at least 35 countries and prevent the reintroduction of malaria in all countries deemed malaria free. According to the World Malaria Report 2016, the rate of new cases fell by 21 percent globally between 2010 and 2015, with death rates dropping by 31 percent over the same period in sub-Saharan Africa.
Better vaccines to come?

Glaxo is not calculating on making profit in this involvment
Developed in the 1980s by GlaskoSmithKline, RTS,S is the first malaria vaccination to undergo a Phase 3 clinical trial, which it did in 2014 involving more than 15,000 infants and youth in seven African countries. In July 2015, RTS,S obtained a positive scientific opinion from the European Medicines Agency, a medicine regulatory authority. The trials are funded by Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; UNITAID; the WHO and GSK. In addition to its own success so far, RTS,S has reinvigorated the hunt for an effective vaccine. “This first generation malaria vaccine gives us hope, but we need to look forward to next generation malaria vaccines as well,” Kaslow said.
Currently second-generation vaccines are being tested that would treat different phases in the parasite’s life cycle. Some even build on the RTS,S vaccination, according to Hamel. “We expect there will be subsequent malaria vaccines that come along that work even better than this one, but those vaccines won’t be available to children for five to 10 years, maybe longer, which is why it’s still worthwhile going through with this vaccine because many lives can be saved in the meantime,” Hamel said. “We finally have a vaccine against malaria and that in itself shows this can be done and that funding and research must continue because it’s possible.”



























