It is emerging that Ebola virus might have capacity to hibernate and return for a second round of infection. According to the New York Times, Scientists seem to conclude that someone who was infected in the 2014 outbreak retained the virus and transferred it through sex five years or so later.

A BBC mapping of the current axis of infection but who can tell what happens tomorrow?
There is still nothing conclusive about this but it is something to worry about if it were to be found to be the case. Unlike Covid – 19, Ebola seems to be a peculiar African public health crisis for now. Right now, Guinea, DRC, Liberia and Sierra Leone are the areas of infection.
According to the paper, the virus whose longest known survival span was 500 days is turning a stunner for health experts who are reportedly calling it “an extraordinary phenomenon”. The new finding is coming from what the New York Times call genetic sequencing of virus samples taken from patients in the current outbreak. The current outbreak is a reference to the current phase of Ebola crisis in the Republic of Guinea in West Africa which has been under siege since January, 2021.
Says NYT: People recover from Ebola when their immune systems wipe out the virus. But certain parts of the body, including the eye, the central nervous system and the testes are so-called privileged sites, beyond the reach of the immune system. The virus can sometimes hide in those spots. But no one knew it could hide out for so long”.
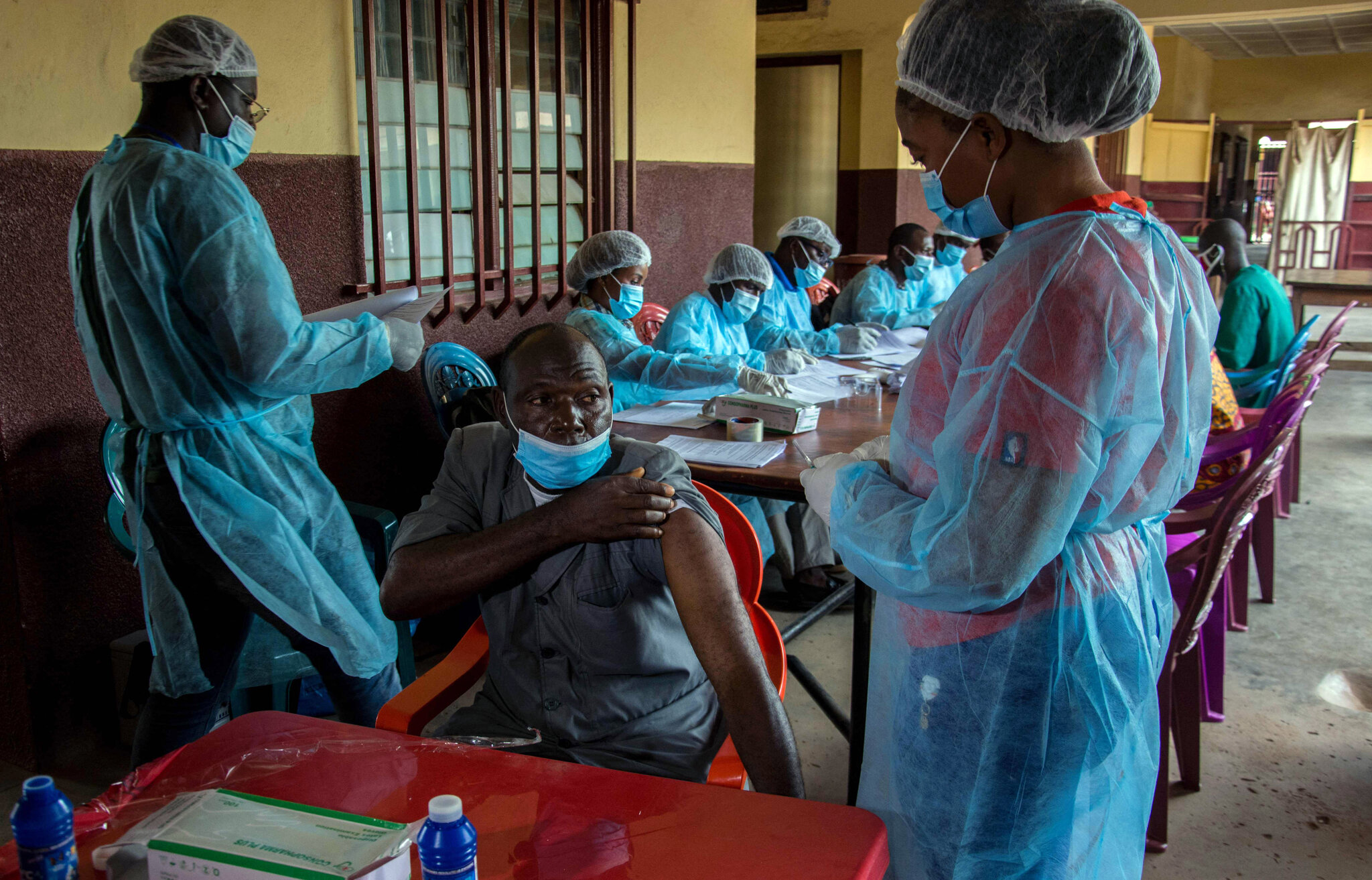
There is a hint that one way out is “to vaccinate much of equatorial Africa” against Ebola even where there has been re-occurrence or an outbreak. While the vaccines are said to be available, the logistics of accomplishing vaccinating much of equatorial Africa could test existing indigenous expertise and infrastructural facilities.
Earlier in January 2021, four leading international health and humanitarian organizations announced the establishment of a global Ebola vaccine stockpile to ensure outbreak response. With this news, it is no longer clear which is which.
According to a widely published statement then, the effort to establish the stockpile was led by the International Coordinating Group (ICG) on Vaccine Provision, which includes the World Health Organization (WHO), UNICEF, the International Federation of Red Cross and Red Crescent Societies (IFRC), and Médecins Sans Frontières (MSF), with financial support from Gavi, the Vaccine Alliance. The stockpile will allow countries, with the support of humanitarian organizations, to contain future Ebola epidemics by ensuring timely access to vaccines for populations at risk during outbreaks.
The statement added that the injectable single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live) is manufactured by Merck, Sharp & Dohme (MSD) Corp. and developed with financial support from the government of the United States of America (USA), adding that the European Medicines Agency licensed the Ebola vaccine in November 2019, and the vaccine is now prequalified by WHO, and licensed by the US Food and Drug Administration as well as in eight African countries.

What becomes of their initiative now?
The vaccine was, before achieving licensure, administered to more than 350,000 people in Guinea and in the 2018-2020 Ebola outbreaks in the Democratic Republic of the Congo under a protocol for “compassionate use”.
Director-General of the World Health Organisation, (WHO), Dr Tedros Adhanom Ghebreyesus had been quoted as saying that “Ebola vaccines have made one of the most feared diseases on earth preventable. This new stockpile is an excellent example of solidarity, science and cooperation between international organizations and the private sector to save lives.”
Are the nice things said at the dawn of the initiative now challenged? Henrietta Fore, UNICEF Executive Director had said in the statement then how proud they were to be part of what she calls “this unprecedented effort to help bring potential Ebola outbreaks quickly under control,” “We know that when it comes to disease outbreaks, preparedness is key. This Ebola vaccine stockpile is a remarkable achievement – one that will allow us to deliver vaccines to those who need them the most as quickly as possible.”
But this was against the background that Ebola outbreaks were relatively rare and unpredictable and there is no natural market for the vaccine. Vaccines are only secured through the establishment of the stockpile and are available in limited quantities. The Ebola vaccine is reserved for outbreak response to protect people at the highest risk of contracting Ebola – including healthcare and frontline workers.
For that reason, it could be said that “This is an important milestone. Over the past decade alone we have seen Ebola devastate communities in West and Central Africa, always hitting the poorest and most vulnerable the hardest,” said IFRC Secretary General, Jagan Chapagain. “Through each outbreak, our volunteers have risked their lives to save lives. With this stockpile, it is my hope that the impact of this terrible disease will be dramatically reduced.”
“The creation of an Ebola vaccine stockpile under the ICG is a positive step”, said Dr Natalie Roberts, Programme Manager, MSF Foundation. “Vaccination is one of the most effective measures to respond to outbreaks of vaccine preventable diseases, and Ebola is no exception. An Ebola vaccine stockpile can increase transparency in the management of existing global stocks and the timely deployment of the vaccine where it’s most needed, something MSF has called for during recent outbreaks in the Democratic Republic of Congo.”
An initial 6890 doses are now available for outbreak response with further quantities to be delivered into the stockpile this month and throughout 2021 and beyond. Depending on the rate of vaccine deployment, it could take 2 to 3 years to reach the SAGE-recommended level of 500,000 doses for the emergency stockpile of Ebola vaccines. WHO, UNICEF, Gavi and vaccine manufacturers are continuously assessing options to increase vaccine supply should global demand increase.
All these statements might now have to be reviewed in the light of the emerging discovery